Compositions of Primitive Magmas and Source Characteristics
of the Hawaiian Plume: Constraints from Picritic Lavas
M.D. Norman1 and M.O. Garcia2, 1GEMOC, School of Earth Sciences,
Macquarie University, North Ryde NSW 2109 Australia, marc.norman@mq.edu.au;
2Geology-Geophysics Dept., University of Hawaii, Honolulu HI 96822
USA, garcia@soest.hawaii.edu
The Hawaiian plume is the largest, hottest, longest lived mantle plume currently active on Earth. Moderately evolved tholeiites (7-8% MgO) are volumetrically dominant but are subject to modification by high-level magmatic processes. To provide better constraints on primitive magma compositions and source characteristics of the Hawaiian plume, we have studied picritic lavas (>15% modal olivine) from 7 volcanoes. Picrites are abundant on the flanks of Hawaiian volcanoes and may sample deeper levels of the magmatic system. Some appear to have escaped the complexities of shallow magma chambers [1,2], and therefore may provide the clearest picture available of primitive magmatic characteristics of the Hawaiian plume.
Picrites in our suite contain 13-30% MgO. Log-log plots of compatible vs. incompatible elements show trends consistent with accumulation of olivine into parental magmas with ~14% MgO, so the least magnesian picrites in our study represent close approaches to parental magma compositions, and include samples from Kilauea, Hualalai, and Koolau.
Common-denominator plots such as Al/Ni vs. 1/Ni, and Al/Mg vs. 1/Mg that include all our picrites as well as more evolved tholeiites show well-defined linear trends that are consistent with two component mixtures of olivine and melt. The tight trends are somewhat surprising considering the range of possible olivine and melt endmember compositions, and suggest that these lavas formed by processes that are common to Hawaiian volcanoes.
Linear regressions for these arrays indicate an Fo88 olivine endmember with 2829 ppm Ni, consistent with the compositions of olivines in these and other Hawaiian picrites [2,3,4]. Fo88 olivine would be in equilibrium with a 12% MgO melt (Kd=0.3, Fe3+/Fe-total =0.1), and regression analysis indicates this melt would contain 449 ppm Ni. A similar analysis for Co indicates 166 ppm in the olivine and 59 ppm in the melt. These compositions correspond to olivine/melt Kd's of 6.3 for Ni and 2.8 for Co, and a Ni/Co of 17.0 in the olivine, similar to experimentally determined values for melts of this composition at fO2 > IW [5].
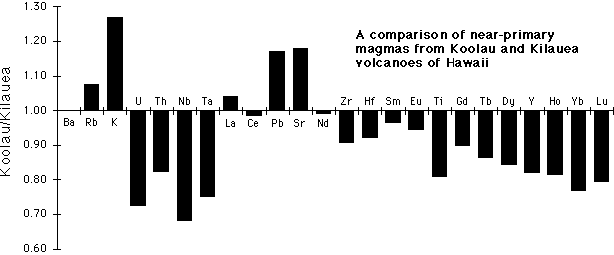
Major element, trace element, and isotopic compositions of Hawaiian lavas demonstrate the presence of at least 2 and possibly 3 distinct components within the Hawaiian plume [6,7]. Fig. 1 compares near-parental lavas from Koolau and Kilauea as an illustration of the trace element characteristics that distinguish these two endmembers. The third endmember is similar in trace element characteristics to Kilauea but can be distinguished by isotopic compositions [6]. Fig. 1 shows that relatively primitive lavas from Koolau are enriched in Sr and Pb abundances, have similar Ba and LREE abundances, and are depleted in Th, Nb, Zr, Hf, Sc and HREE compared to lavas with similar MgO contents from Kilauea.
The increasing depletion of the HREE and Sc, the fractionation of Zr and Hf from the LREE, and the enrichment in Sr and Pb suggest a larger fraction of residual garnet in the source region for Koolau lavas compared to those from Kilauea. Variable amounts of garnet in the Koolau source was also suggested by Frey et al. [8]. Ratios that are sensitive to melting (La/Yb, Sm/Nd, Lu/Hf) suggest that for these picrites, the degree of melting increases over the sequence Loihi, Koolau, Kilauea, Hualalai, Mauna Loa, and is not related directly to isotopically-defined source components. The depletions of Nb, Ti, and Th in the Koolau lavas are difficult to explain by melting, and must be an intrinsic feature of the Koolau component in the Hawaiian plume.
References: [1] Garcia et al. (1989) JGR 94, 10525-10538
[2] Clague et al. (1995) J. Pet. 36, 299-349 [3] Garcia et
al. (1995) In: Mauna Loa Revealed, 219-239 [4] Rhodes (1995) In:
Mauna Loa Revealed, 241-262 [5] Ehlers et al. (1992) GCA 56, 3733-3743.
[6] Hauri (1996) Nature 382, 415-419 [7] Bennett et al. (1996)
Nature 381, 221-224 [8] Frey et al. (1994) GCA 58, 1441-1462

© Copyright Macquarie University | Privacy Statement | Accessibility Information
Site Publisher: DVC Development and External Relations | Last Updated: 18 August 2008
ABN 90 952 801 237 | CRICOS Provider No 00002J