Mark C. Pirlo
GEMOC ARC National Key Centre, Department of Earth and Planetary Sciences, Macquarie University, Sydney, NSW 2109, Australia
Abstract
The Honeymoon Uranium Project in South Australia will use an acid in-situ
leach (ISL) mining technique to recover uranium from mineralised sand aquifers
in Tertiary paleochannels. The geochemical modeling code REACT has
been used to study wastewater arising from the uranium leaching and extraction
plant operations. The preferred disposal option for the wastewater
is re-injection into local aquifers. Mixing reactions involving this
wastewater and natural groundwater have been examined with the model to
estimate the potential for adverse mineral precipitation and environmental
problems. Total mineral precipitation is estimated to be less than
4x10-2g/L, based on a groundwater:wastewater mixing ratio of
10:1. Due to the saline nature of the groundwaters in the region
(ionic strengths range up to 0.4), the modeling has compared the Pitzer
and Debye-Hückel equations for calculating activity coefficients.
Comparable results were obtained from both techniques. These results
lend support to the preferred disposal technique.
1 INTRODUCTION
The Honeymoon Uranium Project is located in South Australia, 75 km
NW of Broken Hill (Figure 1). The region is referred to as the Curnamona
Province, off the Frome Embayment of the Great Artesian Basin.
The Honeymoon deposit is a paleochannel roll-front uranium deposit,
hosted in unconsolidated pyritic, carbonaceous, quartz sand aquifers.
The operators of the Honeymoon Project propose to use a sulfuric acid in
situ leach (ISL) mining technique to recover the uranium, making it only
the second such operation in Australia.
Concerns have been raised regarding the use of acid ISL uranium mining
and wastewater disposal practices at the Honeymoon project e.g. Mudd (1998,
2000). The concerns have included:
- mobilisation of heavy elements by acid solutions;
- increased potential for mineral precipitation (particularly gypsum and jarosite) which (if significant) has potential to cause permeability loss in the formation and excursions of leaching or re-injected wastewater solutions;
- the effect of re-injection of untreated liquid wastes into mineralised aquifers;
- the feasibility of relying upon natural restoration to rehabilitate areas after mining.
Figure 1. Location of the study area (Frome Embayment) in relation
to Broken Hill and South Australia.
2 LEACH PROCESS
The sediments that host the deposit are channel fill deposits in Tertiary
paleochannels which have been incised into the pre-Cambrian basement.
The uranium ore consists predominantly of coffinite that has been adsorbed
onto grains of quartz sand. The ore is present in multiple aquifers
in the Tertiary Eyre Formation (average thickness 50m) which is overlain
by the Tertiary Namba Formation (average thickness 40m) and approximately
30m of Quaternary cover. The ore-grade uranium mineralisation is
generally restricted to the Basal Sand aquifer that directly overlies the
basement. The geology, mineralogy and genesis of the uranium mineralisation
in the Frome Embayment have been described by Callen (1976); Brunt (1978);
Ellis (1980); Giblin (1987) and Southern Cross Resources (2000).
The natural groundwater in the paleochannel aquifers has a predominantly
neutral pH and a reducing redox potential, attributed to the abundance
of pyrite (up to 7%) and carbonaceous material in the aquifer. The
high salinity (TDS = 18,000 mg/L) and high radionuclide content preclude
the direct use of the natural groundwater for any industrial, agricultural
or domestic purpose.
Description of the ISL process has been provided by Lackey (1975) and
Larson (1978). Sulfuric acid will be added to natural groundwater
to reduce the pH from the 6.7-7.7 range down to approximately 2.2.
Oxidants (O2, H2O2 and NaClO3)
will also be added to oxidise the insoluble tetravalent uranium into the
more soluble hexavalent state. Reactions between oxidants and abundant
pyrite in the aquifer also help reduce the total acid requirements of the
leachant.
The Honeymoon Uranium Project will use a 440 L/s wellfield to inject
leaching solution into the orebody. The leaching solution will then
dissolve and leach the uranium from the mineralised sands before being
recovered from extraction wells. An organic solvent extraction plant
on the surface will remove the aqueous uranium species from the leaching
solution before returning the leaching solution to the wellfield.
3 GROUNDWATER MANAGEMENT AND WASTEWATER DISPOSAL
Approximately 1% more water is extracted from the wellfield than is
injected. The result is to draw the surrounding groundwater towards
the central extraction bore in each wellfield and thereby limit excursions
of leaching solutions. This water constitutes the wellfield bleed
stream and represents approximately 376 m3/d when operating at full capacity.
This acid, mildly radioactive wastewater requires disposal. Other
sources of wastewater arising from the ISL plant and mining operations
include: process plant water (115 m3/d), reverse osmosis treatment
brine (60 m3/d) and wellfield development water (115 m3/d).
Total wastewater is estimated at approximately 680 m3/d.
The disposal strategy preferred by the operator is to combine the various
wastewater streams and re-inject them into the basal sand aquifer in areas
that have been mined or in areas away from the existing ore body.
Other than mixing with natural groundwater, no other chemical pretreatment
of the waste stream will take place before re-injection. Natural
restoration at ISL uranium mines in Texas and Wyoming has been predicted
in column experiments (Deutsch 1997), with the critical factor for successful
natural remediation being the amount of pyrite present in the aquifer.
At Honeymoon, the aquifers contain up to 7% pyrite. Mixing and diluting
the acid, oxidising wastewater with neutral, reducing groundwater is inferred
to be sufficient to restore the system.
4 GEOCHEMICAL MODELING APPROACH
The program used to construct geochemical equilibrium and mixing models
is called REACT (Bethke 1996, 1998). REACT is used to predict mixing
products and gauge the ability of natural groundwaters to return to their
natural compositions after injection of a wastewater stream. The
thermodynamic database used by REACT in calculations is from the Lawrence
Livermore National Laboratory (Bethke 1998).
To characterise the baseline chemical composition of natural groundwaters,
samples were collected from monitoring bores in paleochannels containing
uranium mineralisation and analysed for a suite of major and trace elements,
including in-field analyses for a number of parameters (pH, Temp, Conductivity,
DO, Fe(II)).
The modeling approach required development of an equilibrium model
for each natural groundwater. The equilibrium model depicts solution
characteristics, aqueous phase speciation and mineral saturation states
and/or masses of minerals formed. A 10:1 mixing model was then evaluated
where 10 kg of natural groundwater sample was mixed with 1 kg of the wastewater.
Both Pitzer and Debye-Hückel equations were used for calculating
activity coefficients because the natural groundwaters and the wastewater
stream are sufficiently saline (ionic strength approaches 0.4 for wastewater
and some groundwaters), for evaluation of the effect of salinity on the
activity coefficients.
5 RESULTS
Table 1 gives the input parameters (chemical analyses) considered by
the mixing model for 3 natural groundwaters and the wastewater stream.
The composition of the wastewater stream has been determined from analysis
of the current wastewater stream arising from the current field leach trial.
REACT was used to evaluate the results of "mixing" 10 kg of natural
groundwater with 1 kg of the wastewater. Selected results are included
in Table 2.
Of the six natural groundwaters considered, samples H142 and H186 can
potentially form the most
Table 1. Analyses of natural groundwaters from around the Honeymoon Uranium Project. The composition of the wastewater stream is also included.
|
|
|
|
|
|
| pH | 2.8 | 6.67 | 6.88 | 6.99 |
| Units | mg/kg | mg/kg | mg/kg | mg/kg |
| Na+ | 4980 | 3500 | 5000 | 3210 |
| K+ | 27.5 | 18.5 | 21 | 46 |
| Ca++ | 860 | 594 | 1060 | 529 |
| Mg++ | 373 | 300 | 430 | 298 |
| HCO3- | <5 | 167 | 122 | 125 |
| Cl- | 8020 | 6020 | 9240 | 5170 |
| SO4-- | 4190 | 1560 | 1920 | 1800 |
| F- | 1.9 | 0.5 | 0.5 | 0.8 |
| SiO2(aq) | 101 | 14 | 15.6 | 13.5 |
| Units | ug/kg | ug/kg | ug/kg | ug/kg |
| Co++ | 2200 | 80 | 55 | 7 |
| Ni++ | 3530 | 105 | 70 | 563 |
| Ba++ | 77 | 42 | 30 | 38 |
| Pb++ | 17 | 1 | 1 | 3 |
| Cu+ | 1800 | 0.01 | 0.01 | 22 |
| Zn++ | 56300 | 0.03 | 200 | 521 |
| Cr+++ | 100 | 0.02 | 0.02 | 311 |
| Fe+++ | 133000 | 1000 | 1000 | 2890 |
| Mn++ | 400 | 100 | 100 | 68 |
| Al+++ | 28300 | 1000 | 1000 | 10 |
| U++++ | 2530 | 3300 | 22 | 24 |
Table 2. Selected solution characteristics, mineral saturation states and mineral masses/volumes formed by mixing 10 kg of natural groundwater with 1 kg of wastewater.
| Units | H142 | H186 | CMonB | |
| pH | 6.08 | 6.07 | 6.06 | |
| Eh | Volts | -0.08 | -0.08 | -0.08 |
| Ionic strength | Molal | 0.233 | 0.327 | 0.219 |
| TDS | mg/kg | 12600 | 17600 | 11900 |
| Chalcocite | g | 0.002 | 0.002 | 0.003 |
| Kaolinite | g | 0.169 | 0.173 | 0.117 |
| Nontronite-Na | g | 0.143 | 0.098 | 0.191 |
| Quartz | g | 0.018 | 0.063 | 0.019 |
| Uranophane | g | 0.063 | 0.005 | 0.005 |
| Total mineral mass produced | g | 0.418 | 0.360 | 0.361 |
| Totalmineral volume produced | cm3 | 0.151 | 0.135 | 0.136 |
| Gypsum | log(Q/K) | -0.545 | -0.394 | -0.519 |
| Anhydrite | log(Q/K) | -0.718 | -0.565 | -0.692 |
| Calcite | log(Q/K) | -1.26 | -1.26 | -1.46 |
| Dolomite | log(Q/K) | -1.58 | -1.64 | -1.93 |
| Alunite | log(Q/K) | <-3 | <-3 | <-3 |
| Jarrosite | log(Q/K) | <-3 | <-3 | <-3 |
| Nontronite-Mg | log(Q/K) | -0.0106 | -0.0342 | -0.0014 |
and least precipitated mineral mass/volume respectively, when mixed
with the wastewater in a 10:1 ratio (Table 2). The minerals that
form, and in some cases re-dissolve, over the course of the mixing reaction
can be displayed as a mixing reaction trace. Figures 2 and 3 show
mixing reaction traces for these two groundwaters. The vertical axis
shows the mass of each mineral produced, whilst the horizontal axis shows
the cumulative mass of solution. 10 kg of the natural groundwater
is progressively titrated against 1 kg of the wastewater to give a total
solution mass of 11 kg.
Figure 2. Mixing reaction trace between natural groundwater H142
and wastewater. 10 kg of H142 is gradually added to 1 kg of wastewater.
The formation and dissolution of mineral phases can be seen as a function
of reaction progress. Of the three natural groundwaters considered,
H142 produced the largest mineral mass.
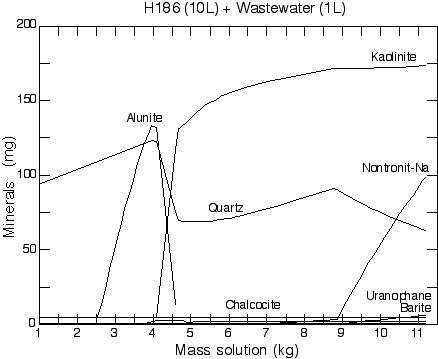
Figure 3. Mixing reaction trace between natural groundwater
H186 and wastewater. 10 kg of H186 is gradually added to 1 kg of
wastewater. The formation and dissolution of mineral phases can be
seen as a function of reaction progress. Of the three natural groundwaters
considered, H186 produced the smallest mineral mass.
Comparisons of the modeling results derived from using the Debye-Hückel
approach, with those derived from using a Pitzer (or virial) approach (Pitzer
1973, 1977) could be made on the basis of solution characteristics and
mineral saturation states.
6 DISCUSSION
After mixing 1 kg of wastewater with a pH of 2.8 with 10 kg of natural
groundwater with a pH of approximately 6.8, the resulting solution attains
a minimum pH of 6.1. This is inferred because free H+ ions are consumed
in mineral precipitation reactions or taken up by aqueous complexes/species.
This demonstrates recovery of the system following disposal of the acid
wastewater. Mixing and reaction with further volumes of natural groundwater
would bring the pH even closer to baseline values.
Mineral precipitation predicted by the model is low, particularly when
considering the high permeability of the formation and the fact that mineral
precipitation from the natural groundwater alone has also contributed to
the predicted values. The predicted values therefore represent a
worst case situation, i.e. they represent the maximum mass that is thermodynamically
possible. Precipitation of gypsum and jarosite should not occur because
the saturation index for these minerals is not exceeded Table 2).
For the samples used, the solution characteristics and precipitated
mineral masses predicted by the model using the virial equations were not
significantly different to those obtained using the Debye-Hückel approach
for calculating activity coefficients.
7 CONCLUSIONS
- 1 Mineral precipitation masses/volumes arising from mixing wastewater with natural groundwater are low.
- 2 Relative to the permeability of the aquifer, the low mineral precipitation is not expected to significantly decrease aquifer permeability to a point where excursions of leaching solutions result
- 3 The pH of the mixed solution is comparable to natural background values and will come even closer with further water-rock interactions.
- 4 Ionic strength of natural groundwater is not high enough to require the use of virial equations when estimating activity coefficients.
ACKNOWLEDGEMENTS
The author would like to acknowledge research grants from the Queen's
Trust for Young Australians, Macquarie University and the ARC GEMOC National
Key Centre. Southern Cross Resources Pty Ltd. is thanked for providing
access to samples and field sites. No financial support was provided
by Southern Cross Resources for this research.
REFERENCES
Bethke, C. M. 1996. Geochemical reaction modeling - concepts
and applications. Oxford University Press.
Bethke, C. M. 1998. The Geochemist's WorkbenchÇ Release
3.0. A user's guide to Rxn, Act2, Tact, REACT and Gtplot. University
of Illinois Hydrogeology Program.
Brunt, D. A. 1978. Uranium in Tertiary stream channels,
Lake Frome Area, South Australia. Proceedings of the Australasian
Institute of Mining and Metallurgy 266: 79-90.
Callen, R. A. 1976. Lake Frome Area - regional geology,
Tertiary stratigraphy and uranium localization. In C. L. Knight (ed),
Economic geology of Australia and Paupa New Guinea, Vol. 1, Metals: 803-808.
Australasian Institute of Mining and Metallurgy, Melbourne.
Deutsch, W. J. 1997. Groundwater geochemistry - fundamentals
and applications to contamination. Lewis Publishers, Boca Raton,
New York.
Ellis, G. K. 1980. Distribution and genesis of sedimentary
uranium near Curnamona, Lake Frome Region, South Australia. The American
Association of Petroleum Geologists Bulletin 64: 1643-1657.
Giblin, A. M. 1987. Applications of groundwater geochemistry
to genetic theories and exploration methods for early Tertiary sediment-hosted
uranium deposits in Australia. Uranium 3: 165-186.
Lackey, J. A. 1975. Solution mining (in situ leaching)
- a literature survey. Australian Mineral Development Laboratories
Bulletin 19: 40-61.
Larson, W. C. 1978. Uranium in situ leach mining in the
United States. Bureau of Mines Information Circular 8777. United
States Department of the Interior.
Mudd, G. M. 1998. An environmental critique of in situ
leach mining: the case against uranium solution mining. http://www.sea_us.org.au/isl/no2isl.pdf
(Acessed 28/3/99). Report prepared for Friends of the Earth (Fitzroy)
and the Australian Conservation Foundation.
Mudd, G. M. 2000. Acid in situ leach uranium mining: 1
- USA and Australia. Tailings and Mine Waste '00, Proceedings of
the Seventh International Conference, Fort Collins, Colorado, USA, January
23-26 2000: 517-526.
Pitzer, K. S. 1973. Thermodynamics of electrolytes.
I. Theoretical basis and general equations. The Journal of
Physical Chemistry 77: 268-277.
Pitzer, K. S. 1977. Electrolyte theory - improvements since
Debye and Hückel. Accounts of Chemical Research 10: 371-377.
Southern Cross Resources Australia Pty Ltd. 2000. Honeymoon
Uranium Project Environmental Impact Statement. Southern Cross Resources
Australia Pty Ltd.

 GEMOC ARC National Key Centre
GEMOC ARC National Key Centre